Brassicaceae pollen analysis
It has never been easier to phenotype the pollen of your lines.
Introduction
Dr. Georg Roell
Hello there, I am Georg Roell, Product Manager at Amphasys. I have been working with the Brassicaceae species for more than 15 years at the farm level and in the seed industry. In this blog post I am happy to share my knowledge specifically on Brassicaceae pollen analysis, gained through my journey as Customer Consultant and Product manager at Amphasys. Let’s discover together why, how to collect and finally use pollen information along the seed production process.
What this Article is About
In this blog post I want to give you an overview about the importance of Brassicaceae pollen analysis. In addition, I want to share the best pollen collection methods, as well as a practical example of how pollen quality can differ between lines and how you can drive the right decisions to improve seed production.
The Brassicaceae plant family is a diverse group of flowering plants that includes many important crops such as vegetables like Broccoli, Cauliflower, Kale, Cabbage, or Canola mainly used as oil crop. One of the distinguishing features of the Brassicaceae family is the structure of their flowers, which are four-petaled and arranged in a cross shape. This makes the pollen collection and analysis quite similar across the plant family and enabled us to develop one crop-specific chip for the whole Brassicaceae family.
Subscribe to our blog!
If you want to make sure to be informed about new articles, subscribe to our blog!
But why Brassicaceae pollen analysis?
As you know, plant breeding harnessed the heterosis effect for a broad variety of crops across the Brassicaceae family which creates plants facilitating a higher yield, greater resistance against pathogens and climatic conditions. These entrails a complex and time-consuming breeding and seed production process due to the ongoing production of hybrid seeds, which necessitates the preservation and maintenance of genetic purity of inbred lines.
Hybrid seed production typically requires harvesting seeds solely from female plants, which demands a larger surface area. Consequently, this results in lower seed yield per unit area when compared to open-pollinated (non-hybrid) varieties, leading to significantly higher seed production costs. In addition, due to the long growth cycle of Brassicaceae, it is important to have a predictable and reliable seed production process in place to reduce seed inventory costs as well as to meet the market demand.
Thus, having a reliable and efficient seed production process is crucial, which entails adequate nicking, stable male sterile lines, and male lines with high pollen quality. In Brassicaceae, proper nicking can be achieved through various methods such as altering planting dates (staggered) or pruning male plants to encourage regrowth.
The quality of pollen, however, is primarily influenced by genotype, environmental factors, and plant nutrition which can lead to strong fluctuation in seed yield. Pollen analysis is therefore a key cornerstone in determining the proper genotype for a specific environment including plant nutrition regime.
How to perform Brassicaceae pollen collection?
To ensure accurate pollen measurement, several factors must be considered. It is important to clarify the purpose of the experiment as this can influence the preparation of samples and interpretation of data. For instance, if your objective is to investigate the impact of heat stress on pollen viability, samples must be collected during the period of stress and the climatic conditions during the entire pre- and flowering period climatic data must be recorded for data evaluation.
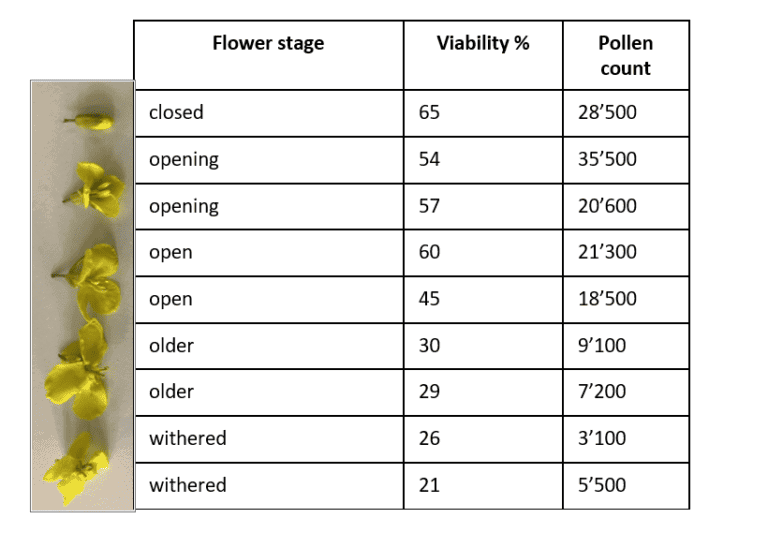
If you want to compare pollen viability and pollen number of different lines, pollen from almost open flowers must be collected (Figure 1). This is especially important, as insects can carry pollen away and the results of pollen amount get distorted. In addition, pollen viability drops after flower opening as it is shown in the following canola experiment executed by us.
In this experiment pollen from different flower stages (Figure 1) (closed, about to open (opening), open, older, and withered) was collected and analyzed. The result of pollen viability and pollen count showed a decreasing trend from closed to withered flowers. While it seems to be obvious that withered flowers contain the lowest pollen viability and pollen number and are not representative for pollen analysis, it is different for open flowers. You can find a higher variance in pollen viability and pollen amount in open and older flowers. Therefore, it is important not to sample just open flowers, but specifically select opening (about to open) flowers to exclude flower aging effect.
How to best extract Brassicaceae pollen?
As we determined the right flower stage for pollen analysis in the paragraph above, different extraction methods were tested to find out which are most suitable for your pollen analysis.
Let’s briefly recap the Brassicaceae flower structure. The brassicaceae flower facilitates six stamens where four of them are longer and two of them are shorter (Figure 2).
For pollen extraction, all stamens of three to five flowers (dependent on the species) must be picked using forceps. They normally contain enough pollen for one measurement. In case you end up with not enough pollen, pick more anthers from additional flowers and transfer them to the Eppendorf tube to proceed with the pollen extraction method.
To determine the best extraction method, we tested two most suitable ones:
- Anther Squeezing
- Pull Over a Rack
Anther Squeezing:
The anther squeezing is a very common procedure for extracting pollen from anthers by gentle squeezing the anthers with a pestle. This method was tested across a variety of Brassicaceae species including applying different pressure when squeezing the anthers. Slightly touching or squeezing of the anthers against the wall of an Eppendorf tube enabled sufficient pollen extraction. Squeezing the anther too much resulted in a high amount of destroyed cells also called debris as indicated in Figure 3 C.
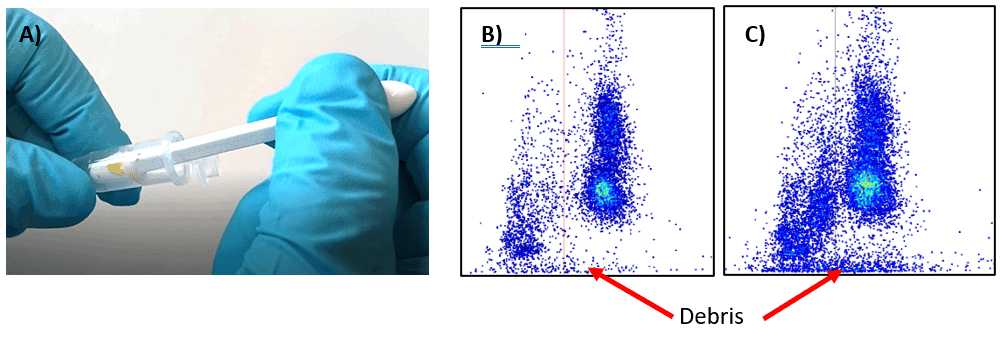
The high number of debris is one of the downsides of this method, especially if this method is performed by multiple users. Due to different squeezing behavior between users the results may be distorted or not representative. In case this method is applied it is important that the extraction step is executed always by the same person to avoid any sampling effect induced by the user. Due to this tradeoff, this method is not recommended for routine pollen measurements, especially in conjunction with our crop-specific chip.
However, this method can be useful for other applications like analyzing microspore developmental stages our Ampha Z40 in double haploid production. More information on this topic will be covered in an upcoming double haploid pollen expert blog.
Pull Over a Rack:
In order to develop a more robust, fast and easy pollen extraction from flowers, another method was evaluated. The “Pull Over a Rack” method is already established and used for other crops like Tomato or Pepper.
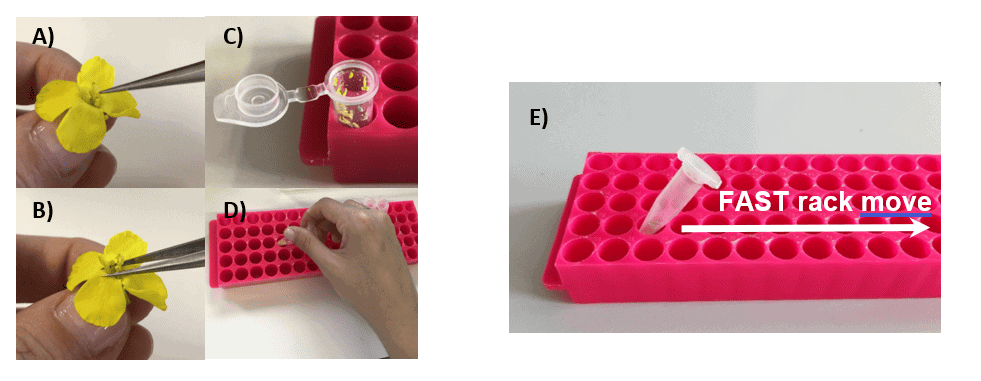
After picking and transferring the anthers in an Eppendorf tube, the tube will be closed and moved five times over the rack as indicated in (Figure 4 A). This method enables gentle pollen extraction without damaging them.
Furthermore, our AmphaFluid AF10 will be added for pollen extraction. This method enables gentle pollen extraction without damaging them.
The amount of debris you are creating will be much lower compared to the anther squeezing method (Figure 3 B; Figure 4 B). This enables easier data analysis and is ideal for routine measurements where several people in parallel can extract the pollen.
How to apply pollen analysis for routine line screening?
To ensure a quick and user-friendly workflow from pollen collection to data analysis, it is crucial to have a suitable method for routine line screening. With this in mind, we have created a crop-specific chip for Brassicaceae that provides clear and easy-to-follow sample instructions and automated data analysis, resulting in immediate results. The Ampha P20, our portable instrument, makes it possible to bring the lab to the field and obtain data that is ready to use back at the office. This development has greatly simplified Brassicaceae pollen analysis.
As demonstrated in a broccoli pollinator test trial (Figure 3), various male lines were analyzed using the Ampha P20 instrument and the newly designed crop-specific chip for Brassicaceae. All male lines were grown under identical environmental conditions, and their pollen was collected during the primary flowering period.
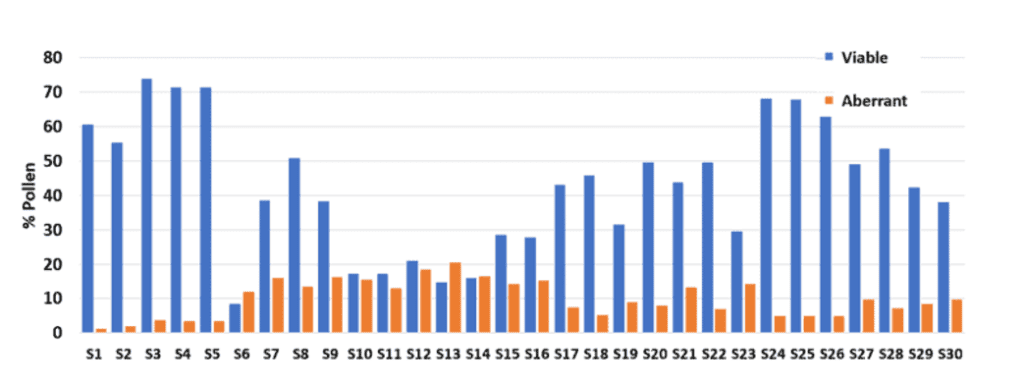
The measurements showed strong difference in pollen viability between the lines ranging from 9% – 75%. These measurements provide further insight into lines with potential pollination issues. These findings indicated lines that may cause pollination challenges, particularly those with pollen viability below 40%. To prevent seed production issues, it is recommended to investigate the root cause of low pollen viability in these lines thoroughly. If feasible, it is recommended to eliminate the lines with low pollen viability to ensure a cost-effective and dependable seed production process.
Furthermore, what the experiment showed, is the high amount of aberrant pollen grains in specific lines. In general, aberrant pollen grains are being formed due to pollen abortion during pollen development. There are several reasons like genetics and biotic or abiotic stress, why the aberrant pollen fraction may be increased. The most common reason is due to heat or drought stress during pollen development. It is very important to know the susceptible lines and how they behave differently under stress conditions. This knowledge can be used to better plan your crop placement to avoid stress scenarios or to exclude challenging lines from your seed production process.
Selecting lines with high pollen viability and growing them under low stress conditions contributes to better reaching the production target in a more reliable way.
Take home message:
Amphasys offers a straightforward routine tool to analyze Brassicaceae pollen to improve seed production efficiency and reliability. The goal is to identify high pollen viability lines and stress tolerance to optimize crop placement according to suitable climatic conditions and avoid stress scenarios.
Since the flower structure is similar across various Brassicaceae species, a single sample preparation protocol can be utilized for the entire Brassicaceae family. The most effective preparation method identified is the “pulling over the rack” technique, which has been extensively tested.
When combined with our specially designed Brassicaceae chip, this method is ideal for conducting high throughput line screening to identify the best pollinator lines. After each measurement, you will receive immediate results, which will help you choose the most suitable lines. This will help you to increase your seed production efficiency and improve reliability.
Thank you very much for staying until here with me. If you have any questions about the application or would like to learn more about how we can assist you in selecting the right trial design, please don’t hesitate to contact me at georg.roell@amphasys.com.
Stay tuned.
Best,
Georg
